What is Nuclear Physics?
Nuclear physics is a branch of physics that delves into the properties, structure, and behavior of atomic nuclei—the dense region at the center of an atom. It encompasses the study of nuclear reactions, including fusion, fission, and radioactive decay, and explores how the nucleus interacts with the rest of the atom and with other nuclei. This field is fundamental to understanding the composition of matter and the vast energy potential within nuclei.
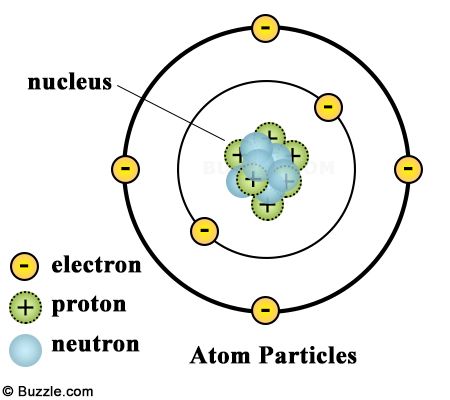
Atomic Structure
Atoms are the building blocks of matter, each consisting of a dense nucleus surrounded by a cloud of electrons. The nucleus, composed of positively charged protons and neutrally charged neutrons, accounts for nearly all the mass of an atom. Electrons, much lighter in mass, occupy the space around the nucleus in regions called orbitals, determining the atom's chemical properties. The balance between the electrostatic forces of attraction and the strong nuclear force within the nucleus dictates the stability of an atom.
Radioactive Decay
Radioactive decay is a natural process by which an unstable atomic nucleus releases energy to form a more stable configuration. This can occur through various modes, notably alpha decay, beta decay, and gamma emission. In alpha decay, the nucleus emits an alpha particle (two protons and two neutrons), slightly reducing its mass and changing its identity. Beta decay involves the transformation of a neutron into a proton (or vice versa), emitting a beta particle (electron or positron) and an antineutrino (or neutrino). Gamma emission releases excess energy from the nucleus without changing its number of protons or neutrons.
Alpha decay example (Uranium-238 to Thorium-234): \( ^{238}_{92}\textrm{U} \rightarrow ^{234}_{90}\textrm{Th} + ^{4}_{2}\textrm{He} \)
Beta decay example (Carbon-14 to Nitrogen-14): \( ^{14}_{6}\textrm{C} \rightarrow ^{14}_{7}\textrm{N} + e^{-} + \bar{\nu}_{e} \)
Decay Rate Visualization for Carbon-14
The decay rate of Carbon-14, a radioactive isotope used in radiocarbon dating, illustrates the concept of half-life—the time required for half of the radioactive atoms in a sample to decay. Carbon-14's half-life of about 5,730 years makes it invaluable for dating archaeological and geological samples.
Applications of Nuclear Physics
Nuclear physics has revolutionized many aspects of our world, offering both profound benefits and formidable challenges. Its applications span from medical treatments and diagnostics, such as cancer therapy with radiation and diagnostic imaging with PET scans, to the generation of electricity in nuclear power plants through the controlled release of nuclear energy. Additionally, nuclear physics principles underpin much of our scientific research, enabling the discovery of new elements, the study of the cosmos, and the pursuit of understanding fundamental particles and forces.
- Medical Imaging and Treatments: Uses radiation to diagnose and treat diseases, enhancing both longevity and quality of life.
- Energy Production: Nuclear reactors generate electricity through fission, providing a potent source of low-carbon energy.
- Scientific Research: Particle accelerators and detectors allow scientists to explore the properties of subatomic particles, contributing to our understanding of the universe.